Identify From the Following Compounds Which One Is Antiaromatic.
Show the steps necessary to transform the compound on the left into the compound on the. So option B is correct.

Among The Above The Number Of Aromatic Compound S Is Are
From the above compounds which satisfy this condition is called the anti -aromatic compound.
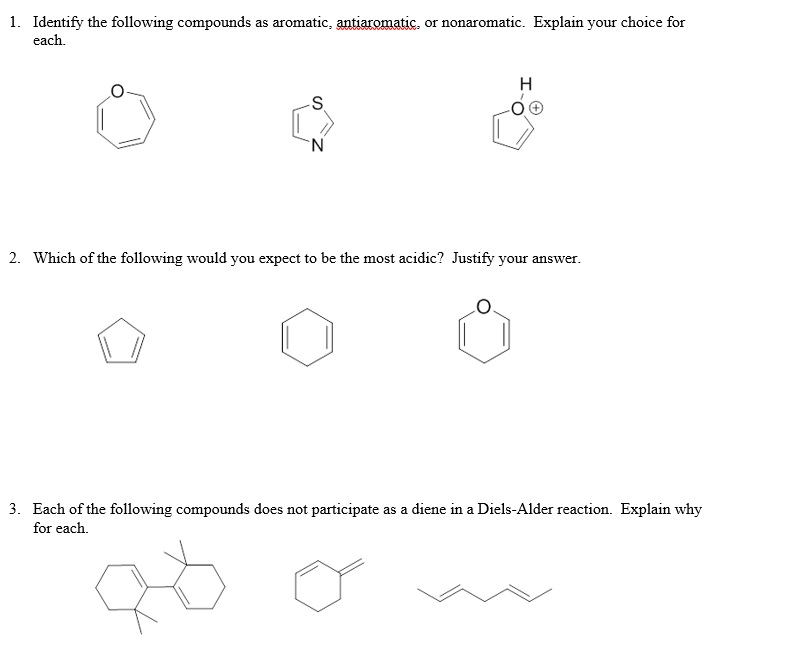
. Cyclic planar compounds with fully conjugated 4n π electrons. It is antiaromatic if all of this is correct except it has 4n electrons Any deviation from these criteria makes it non-aromatic. If has 6 delocalised electrons.
It should be cyclic planar and conjugated system and also follows Huckels rule. Give an explanation for your answer. Pyridine is less basic than triethylamine because AIIMS-2005 1 Pyridine has aromatic character 2 Nitrogen in pyridine is sp 2 hybridized 3 Pyridine is a cyclic system 4 In pyridine lone pair of nitrogen is delocalized.
Which one of the following compound is bartleby. Non- aromatic compounds have 4n2 or. A compound may be aromatic if it obeys the following rules The molecule must be co-planar Complete delocalization of π electron in the ring.
These are antiaromatic compounds. Presence of 4n2 π electrons in the ring where n is an integer n012This is known as Huckels rule. An annulene is a system of conjugated monocyclic hydrocarbons.
Give a brief explanation for your answer. The molecule must be cyclic. First week only 499.
Have 4n2 πe and system should be planar where n is an integer. Il aromatic O I aromatic. The compounds should possess a 4n number of pi electrons where n 012.
View the full answer. A compound is considered anti-aromatic if it follows the first two rules for aromaticity 1. Solution for Which of the following structures if flat would be classified as antiaromatic.
A compound is said to the anti-aromatic if it is cyclic planar have the hybridization as s p 2 and obeys the 4 n π e s rule in which n can be any integer such as 123---and so on. II nonaromatic nonaromatic. Cyclooctatetraene is showing some extra behaviour to avoid Anti-aromaticity.
The last one is very tricky N O N H N H 10e- aromatic 12e- antiaromatic 6e- aromatic the oxygen contribues 2 e- 10e- aromatic the nitrogen contributes 2 e- nonaromatic sp3 carbon 12e- antiaromatic nonaromatic. QUESTION 1 Identify from the following compounds which one is antiaromatic. Anti- aromatic compounds are unstable.
Conditions for compounds to be anti-aromatic. 1 11 IV V IL III IV OV QUESTION 2 Identify from the following compounds those having conjugated double bonds. Pi bonds are in a cyclic structure and 2.
V and VI both have 4e and planar structure. Being planar and cyclic structure completely conjugated system. The molecule has to be planar.
So one difference brings a new class of compounds that are structurally very similar to aromatic compounds however their properties and stability are on the exact opposite of the spectrum. Which of the following species are antiaromatic. Which one of the following compounds is most acidic -AIPMT-2005 1 OH NO 2 2 OH CH 3 3 OH 4 ClCH 2 CH 2 OH 9.
Furan has a planar ring structure. A molecule is aromatic if it is cyclic planar completely conjugated compound with 4n 2 π electrons. Identify the excess reactant and how much of it remains after the reaction of 9.
The compound must be monocyclic. The molecule must be conjugated ie every atom in the molecule should be sp2 hybridized. 18 points For each of the compounds below identify the approximate chemical shift and multiplicity for the circled hydrogen atoms that will appear in the.
II O BI OC. It looks like a perfect student to be named as Anti-aromatic compound cyclic planer fully conjugated 8 pi electrons obeys 4n rule earlier I said that the case is different. The compound must have a conjugated system with a p orbital at every vertex D.
11 111 IV II and IV I III and IV O II IV and V I and III Il and V QUESTION 3 Identify the structure for 12-addition product for the following reaction. I-Z CH3 B IV V O III O IV OT OV O II For the following molecules classify them as aromatic antiaromatic or nonaromatic. For compound to be aromatic it should have 4 n 2 π e.
Labled as A and Identify number of compounds in which ring B is more active than ring A for e asked Dec 13 2019 in Chemistry by Riyanshika 805k points. 15 points Label each of the following compounds as aromatic antiaromatic or nonaromatic. Antiaromatic compounds are molecules that are cyclic planar and completely conjugated but consist of 4n pi electrons.
H H III IV I II O A. How do you identify antiaromatic compounds. II antiaromatic 1 aromatic.
The compound must satisfy Hückels rule -must have 4n 2 electrons. More than one is. Up to 10 cash back It is also important to note that Huckels Rule is just one of three main rules in identifying an aromatic compound.
A I and II B II and III C III and IV D I and IV Can u help me here i think the ans is B since aromatic compounds have a 4n2pi electrons in their ring according to the Huckels rule but is there any other rule so as to find the anti - aromatic compound and if so pls specify. 加工 II I nonaromatic. Start your trial now.
Which one of the following statements is not true for a compound to be considered as aromatic. Which one of the following compound is antiaromatic. Classify the following molecules as aromatic antiaromatic or nonaromatic.
To summarize both aromatic and antiaromatic compounds are cyclic planar and. 11 antiaromatic O I. An Excellent example for this is Cyclooctatetraene.
Identify from the following compounds which one is aromatic. Nonaromatic compounds are molecules that lack one or more of the requirements to be aromatic. Each of the compounds shows below has two aromatic ring.
The compound must be cyclic and planar B.
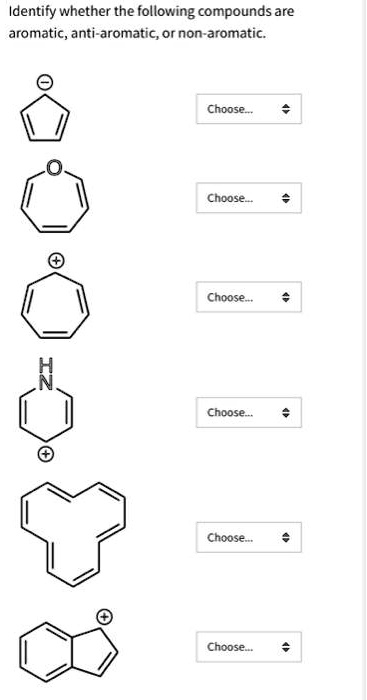
Solved Identify Whether The Following Compounds Are Aromatic Anti Aromatic Or Non Aromatic Choose Choose Choose Caoose Choose Choose

Which Compound Is Antiaromatic And Why Which Of The Following Compounds Is Antiaromatic Image Src Anitaromic8148297908951736195 Jpg Alt Antiaromic Caption Study Com

Classify The Following Compounds As Aromatic Antiaromatic Or Nonaromatic Explain Your Choice Image Src Comp7081280485803489074 Jpg Alt Comp Caption Study Com
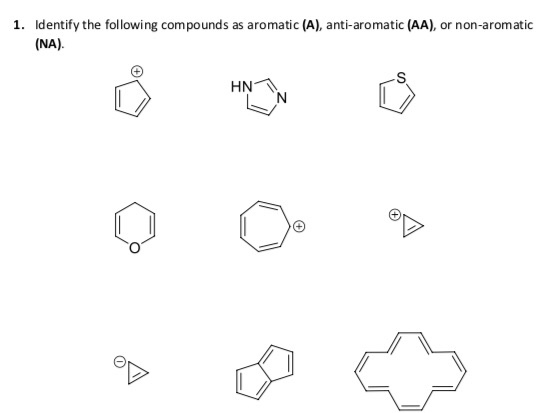
Solved 1 Identify The Following Compounds As Aromatic A Chegg Com
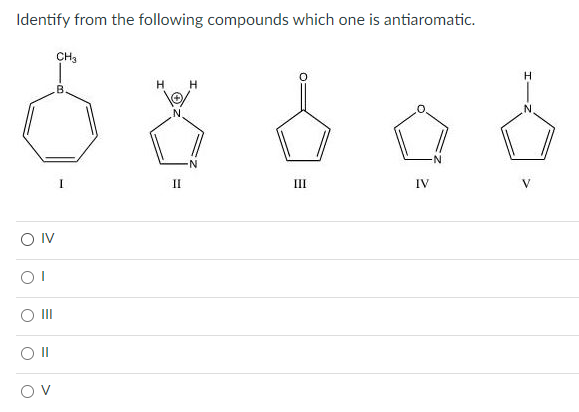
Solved Identify From The Following Compounds Which One Is Chegg Com

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps
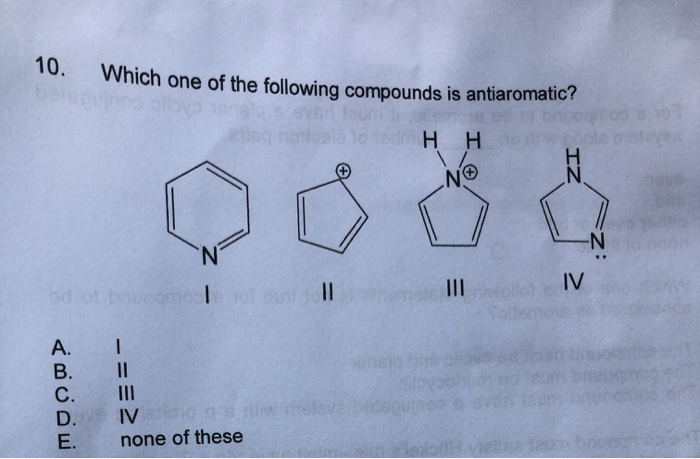
Solved 10 Which One Of The Following Compounds Is Chegg Com
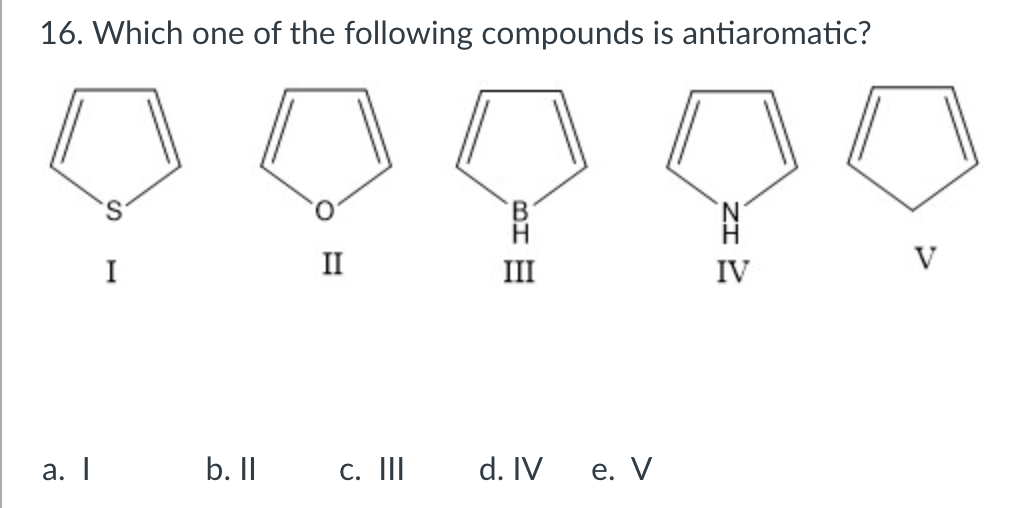
Solved 16 Which One Of The Following Compounds Is Chegg Com

The Given Compound Is Chemistry Questions
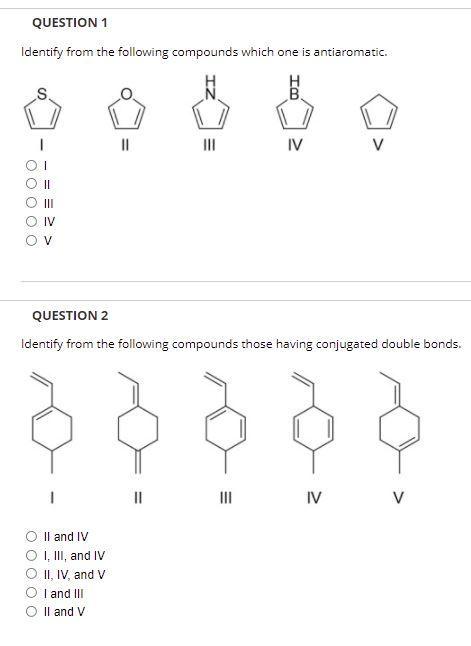
Solved Question 1 Identify From The Following Compounds Chegg Com

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps
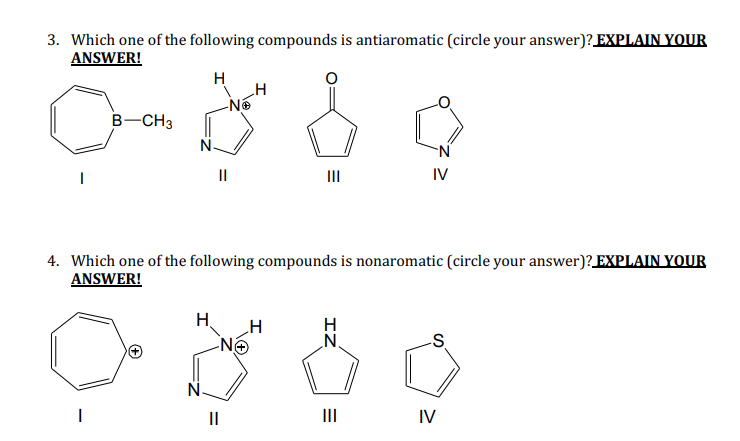
Solved 3 Which One Of The Following Compounds Is Chegg Com
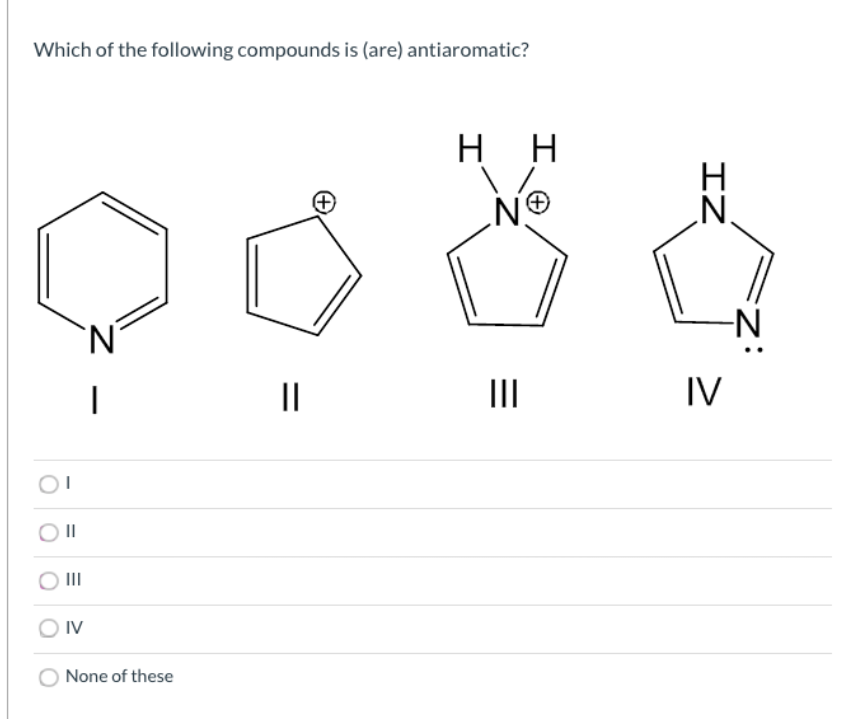
Answered Which Of The Following Compounds Is Bartleby
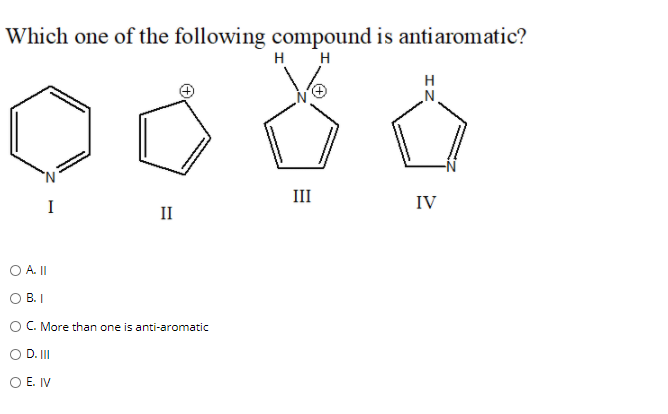
Solved Which One Of The Following Compound Is Antiaromatic Chegg Com
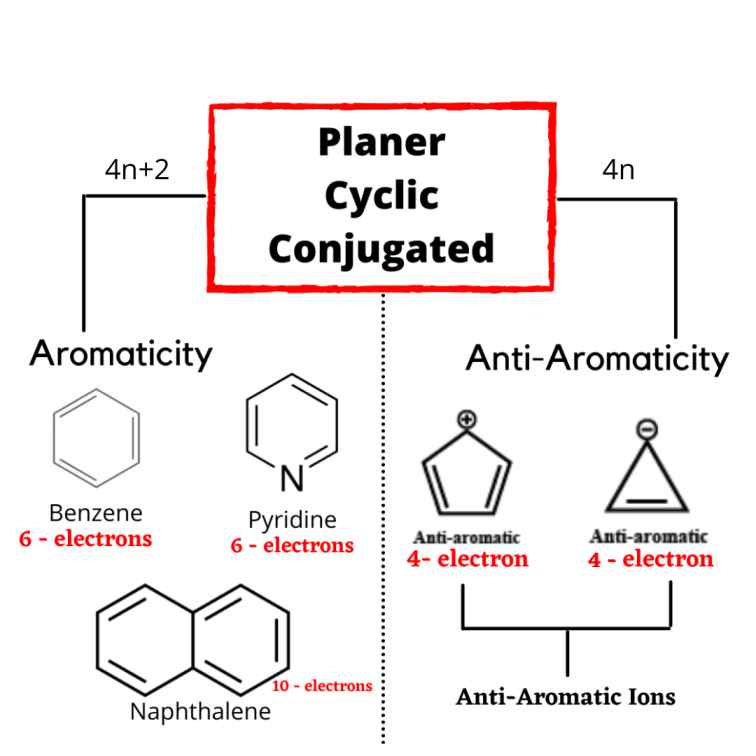
How To Identify Aromatic Anti Aromatic Non Aromatic Compounds
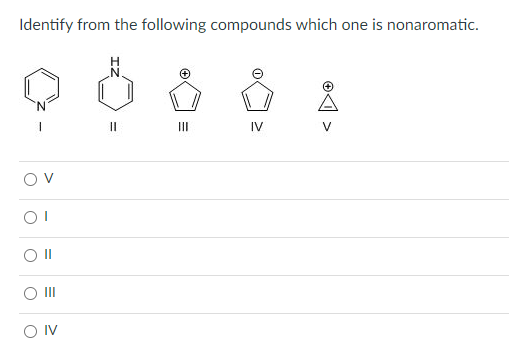
Solved Identify From The Following Compounds Which One Is Chegg Com

Solved 1 Identify The Following Compounds As Aromatic Chegg Com
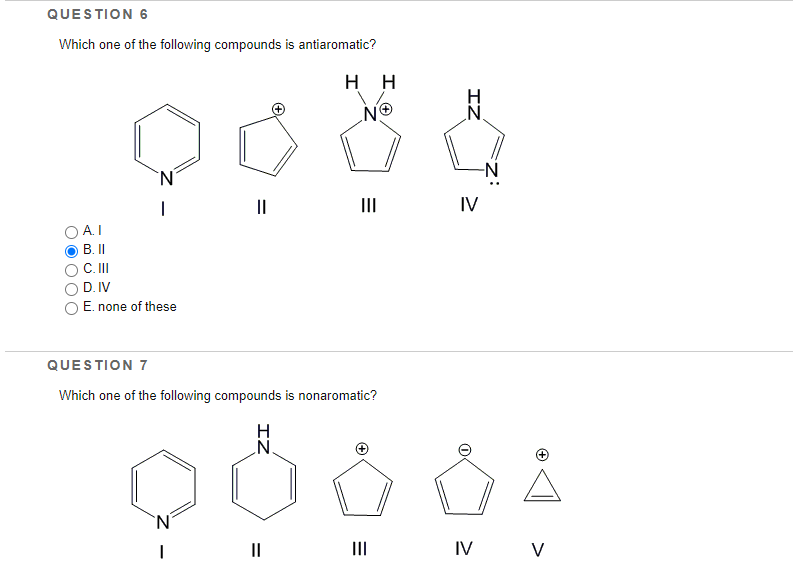
Solved Question 6 Which One Of The Following Compounds Is Chegg Com
Comments
Post a Comment